Digital Oxygen also supports PECAN listing in France
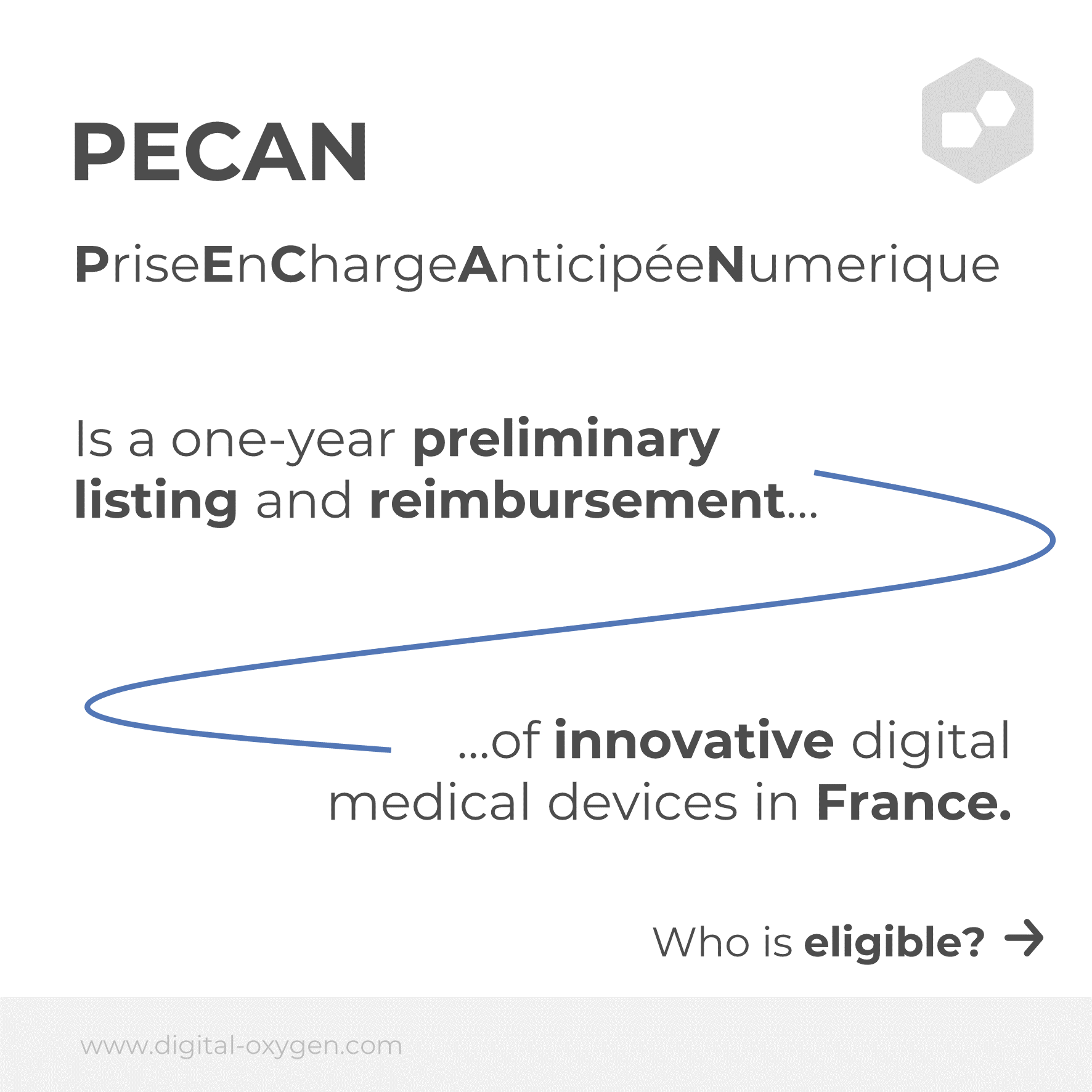
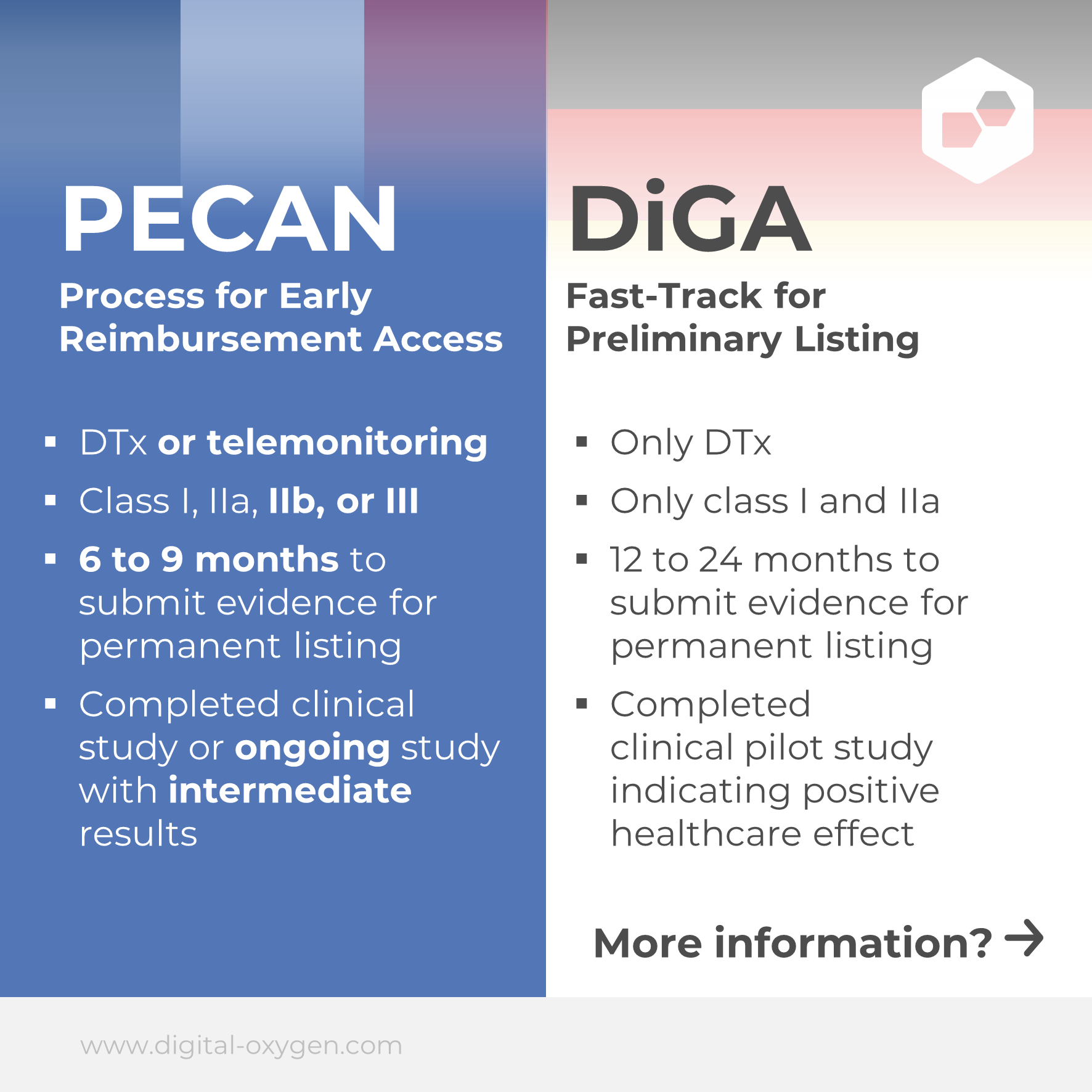
PECAN (Prise en Charge Anticipée Numerique) is the French equivalent of the German fast-track procedure for DiGA approval and provides a fast track to reimbursement of digital medical devices in France.
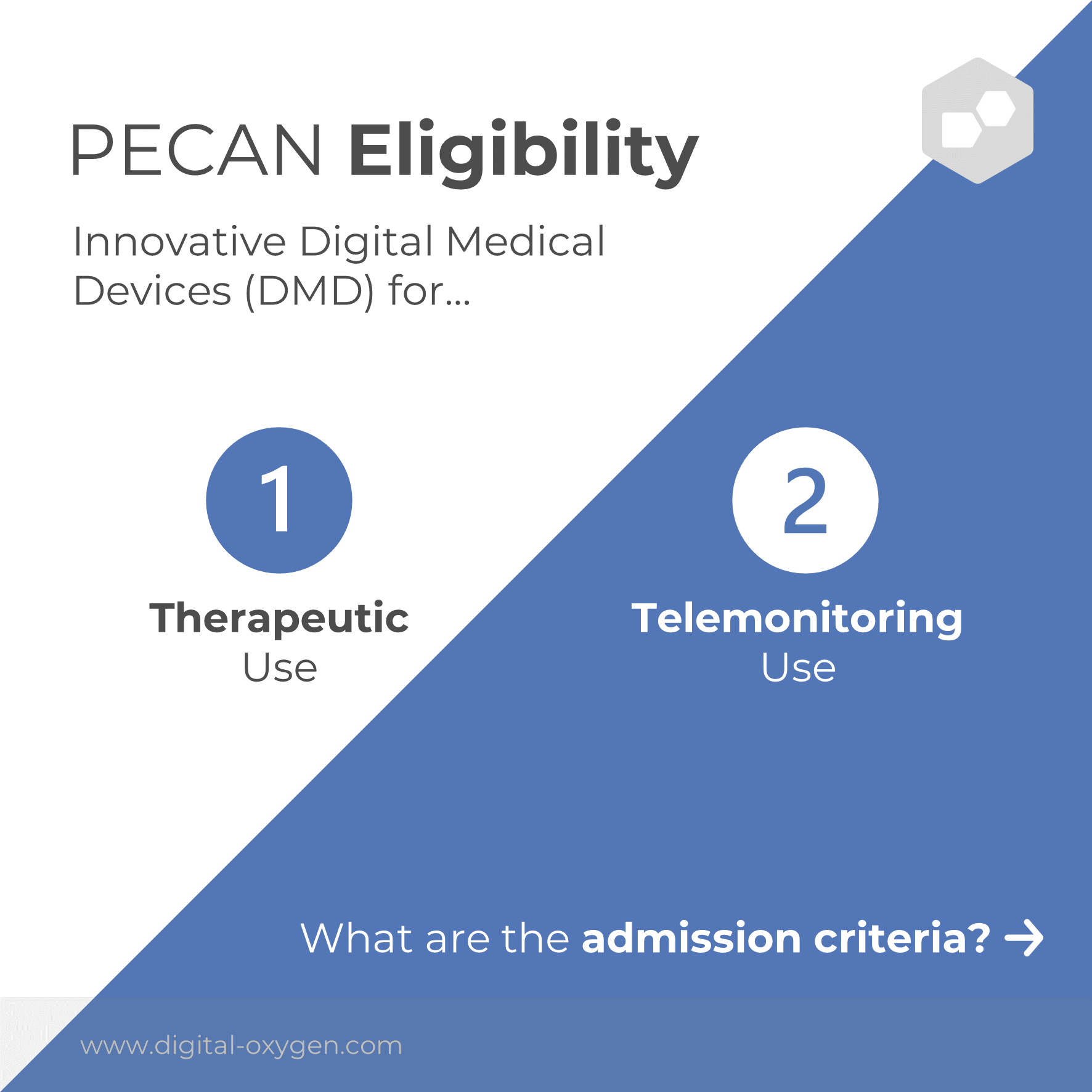
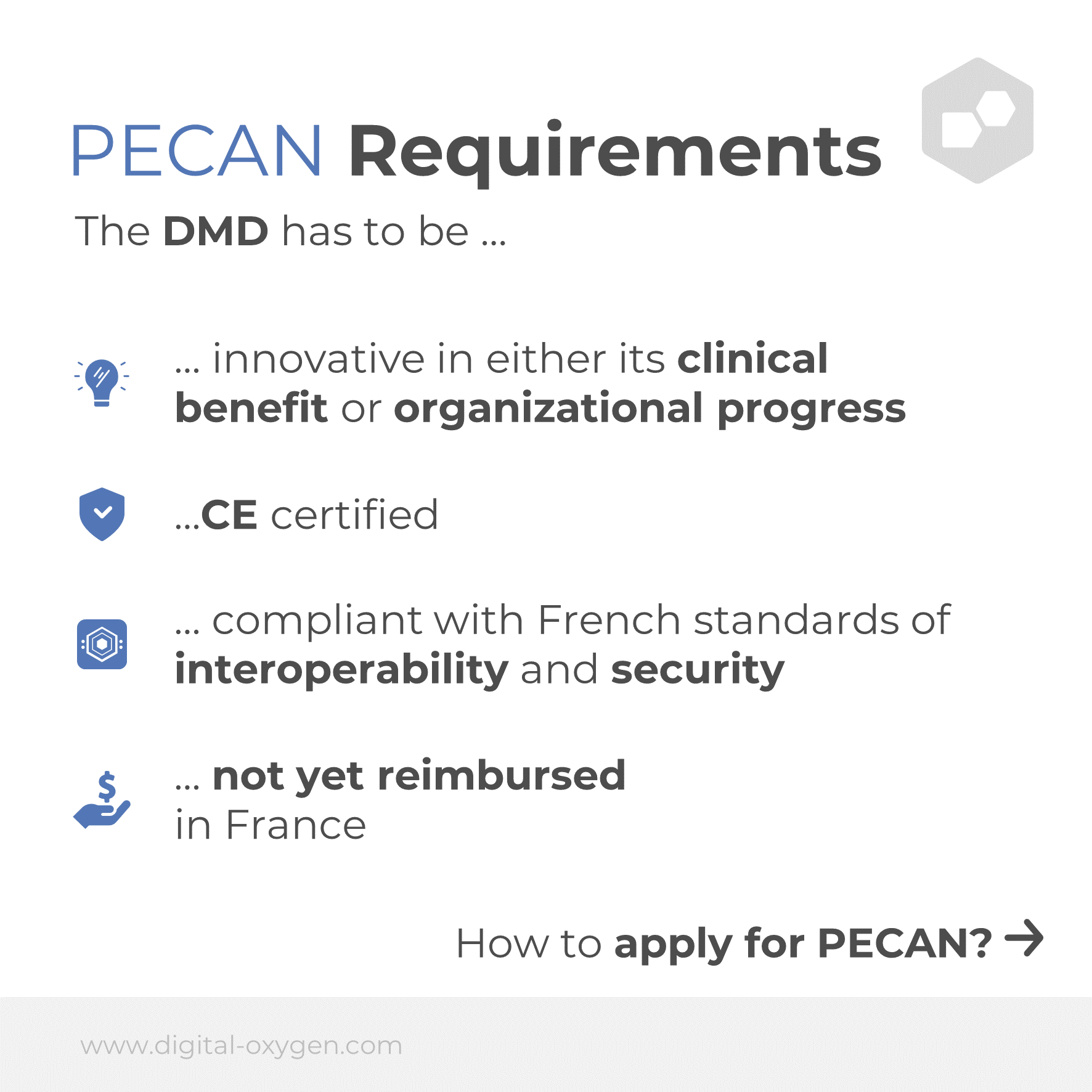
In contrast to the German procedure, PECAN is not only possible for therapeutic applications, but also for telemedical applications. PECAN also has additional requirements: the digital medical device must be innovative in nature, CE certified, compatible with French standards for interoperability and security in digital healthcare, and not yet reimbursed in France.
Three different documents must be submitted for the application:
- A certification of interoperability and security
- A dossier of medical and technical specifications
- A dossier with economic information
With our expertise in DiGA approval and our international network, we support our customers in all issues related to the PECAN process.